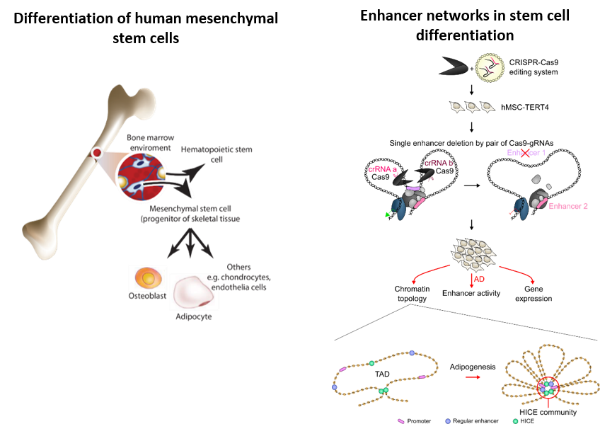
In this project, the Mandrup Group investigates how the 3D chromatin structure and the crosstalk between regulatory elements in the genome determine lineage fate decisions of mesenchymal stem cells, in particular how this drives commitment and differentiation of the adipocyte lineage. We have shown that there is significant crosstalk between enhancers and that these form communities of highly interconnected enhancers that appear to play a role in driving differentiation. We study the molecular mechanisms of this crosstalk including the role of individual enhancers as well as the role of architectural proteins. We are partners in the Novo Nordisk Foundation Center for Genomic Mechanisms of Disease at the Broad Institute and collaborate closely with the researchers at the Broad Institute to discover how genome variation near adipocyte genes affect transcriptional output and adipocyte function.
Technologies: We use cell culture models as well as primary cells combined with a range of sequencing-based functional genomics technologies and bioimaging to map chromatin architecture as well as functional properties of regulatory elements in DNA.
This project is supported by grants from The Novo Nordisk Foundation, Independent Research Fund Denmark, and EU.
People involved
 |
Esra Durmaz Mitchell Postdoc |
 |
Vallari Shukla Postdoc |
 |
Anna Cetnarowska Postdoc |
 |
Marcus Nygård PhD student |
 |
Oliver Bonde van Zwol Research Assistant |
 |
Matthias Gandrup MSc student |
 |
Tobias Jensen MSc student |
Relevant publications
V. Shukla, A. Cetnarowska, M. Hyldahl, S. Mandrup (2022) Interplay between regulatory elements and chromation topology in cellular lineage determination. Trend Genet. Online ahead of print. https://doi.org/10.1016/j.tig.2022.05.011
M. S. Madsen, M. F. Broekema, M. R. Madsen, A. Koppen, A. Borgman, C. Gräwe, E. G. K. Thomsen, D. Westland, M. E. G. Kranendonk, M. G. Koerkamp, N. Hamers, A. M. J. J. Bonvin, J. M. R. Pittol, K. N. Natarajan, S. Kersten, F. C. P. Holstege, H. Monajemi, S. W. C. van Mil, M. Vermeulen, B. B. Kragelund, D. Cassiman, S. Mandrup, E. Kalkhoven (2022) PPARγ lipodystrophy mutants reveal intermolecular interactions required for enhancer activation. Nat Commun. Nov 19;13(1):7090. doi: 10.1038/s41467-022-34766-9.
L. O. Huang, A. Rauch, E. Mazzaferro, M. Preuss, S. Carobbio, C. S. Bayrak, N. Chami, Z. Wang, U. M. Schick, N. Yang, Y. Itan, A. Vidal-Puig,M. den Hoed, S. Mandrup, T. O. Kilpeläinen, R. J. F. Loos (2021) Genome-wide discovery of genetic loci that uncouple excess adiposity from its comorbidities. Nat Metab. 3(2), 228-243.
J. G. S. Madsen, M. S. Madsen, A. Rauch, S. Traynor, E. L. V. Hauwaert, A. K. Haakonsson, B. M. Javierre, M. Hyldahl, P. Fraser & S. Mandrup (2020) Highly interconnected enhancer communities control lineage-determining genes in human mesenchymal stem cells. Nature Genetics. https://doi.org/10.1038/s41588-020-0709-z
A Rauch, A.K. Haakonsson, J.G.S. Madsen, M. Larsen, I. Forss, M.R. Madsen, E.L. Van Hauwaert, C. Wiwie, N.Z. Jespersen, M. Tencerova, R. Nielsen, B.D. Larsen, R. Röttger, J. Baumbach, C. Scheele, M. Kassem, S. Mandrup (2019) Osteogenesis depends on commissioning of a network of stem cell transcription factors that act as repressors of adipogenesis. Nature Genetics 51, 716–727.
A. Loft*, I. Forss*, S. Mandrup (2017) Genome-wide insights into development and function of thermogenic adipocytes. Trends Endocrinol. Metab. 28,104-120.
R. Siersbæk*, J.G.S. Madsen*, B.M. Javierre*, R. Nielsen*, E.K. Bagge, J. Cairns, S.W. Wingett, S. Traynor, M. Spivakov, P. Fraser¤, S. Mandrup¤ (2017) Dynamic rewiring of promoter-anchored chromatin loops during adipocyte differentiation. Mol. Cell 66, 420-435.
A. Loft, I. Forss*, M.S. Siersbæk*, S.F. Schmidt, A.-S. B. Larsen, J.G.S. Madsen, D. Pisani, R. Nielsen, M.M. Aagaard. A. Mathison, M.J. Neville, R. Urrutia, F. Karpe, E.-Z. Amri, S. Mandrup (2015) Browning of human adipocytes requires KLF11 and reprogramming of PPARγ super-enhancers. Genes & Dev. 29, 7-22. (* equal contribution)
R. Siersbæk, S. Baek, A. Rabiee, R. Nielsen, S. Traynor, N. Clark, A. Sandelin, O.N. Jensen, M.-H. Sung, G.L. Hager, S. Mandrup (2014) Molecular architecture of transcription factor hotspots in early adipogenesis. Cell Reports, 7, 1434–1442.
R. Siersbæk, A. Rabiee, R. Nielsen, S. Sidoli, S. Traynor, A. Loft, L.L.C. Poulsen, A. Rogowska-Wrzesinska, O.N. Jensen, S. Mandrup (2014) Transcription factor cooperativity in early adipogenic hotspots and super-enhancers. Cell Reports, 7, 1443–1455.
M.S. Siersbæk*, A. Loft*, M. M. Aagaard*, R. Nielsen, S. F. Schmidt, N. Petrovic, J. Nedergaard, S. Mandrup (2012) Genome-wide profiling of PPARγ in primary epididymal, inguinal and brown adipocytes reveals depot-selective binding correlated with gene expression. Mol. Cell Biol. 32, 3452–3463.
R. Siersbæk, R. Nielsen, S. Mandrup (2012) Transcriptional networks and chromatin remodeling controlling adipogenesis. Trends Endocrinol. Metab. 23, 56-64. (Featured article, front cover, and rated among top ten articles of the year)