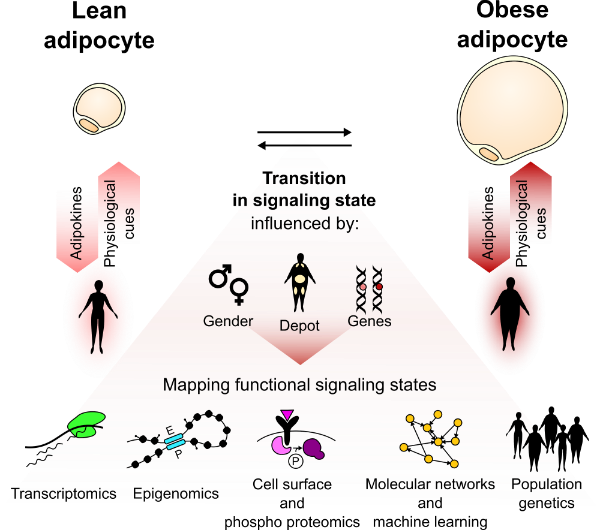
In the context of the Center for Adipocyte Signaling (ADIPOSIGN), the Mandrup Group investigates how adipocytes receive and respond to signals at the level of the transcriptome and epigenome and how these signals differ between adipocytes from different depots, sex and metabolic health. Adipocytes are highly plastic cells with an enormous capacity to adapt to the metabolic need of the body. Several lines of evidence have indicated that the ability of adipocytes to adapt and maintain basic metabolic- and signaling functions is essential for metabolic health. Thus, loss of adipocyte function in obesity is considered a primary driver of obesity comorbidities. We study how changes in signaling states of adipocytes during development of obesity contribute to the adaptation of adipocytes as well as the pathophysiological consequences of obesity.
Technologies: We isolate adipocytes from human subjects with a wide range of BMI and metabolic health and from mouse models challenged with obesogenic diet and use functional genomics technologies to study gene regulatory networks and how these are affected by metabolic signals.
This project is supported by a grant from The Novo Nordisk Foundation.
People involved
 |
Marleen B. Dommerholt Postdoc |
 |
Morgane Philippe Postdoc |

|
Linda Gimm PhD student |
 |
Oliver Bonde van Zwol Research Assistant |
 |
Freya L. H. Rasmussen BSc student |
 |
Trine F. Timmermann BSc student |
Relevant publications
L. K. Markussen, S. Mandrup (2023) Adipocyte gene expression in obesity - insights gained and challenges ahead. Curr Opin Genet Dev. 16;81:102060. doi: 10.1016/j.gde.2023.102060.
L. K. Markussen, E. A. Rondini, O. S. Johansen, J. G. S. Madsen, E. G. Sustarsic, A-B. Marcher, J. B. Hansen, Z. Gerhart-Hines, J. G. Granneman, S. Mandrup (2022) Lipolysis regulates major transcriptional programs in brown adipocytes. Nat Commun 13, 3956.
L. O. Huang, A. Rauch, E. Mazzaferro, M. Preuss, S. Carobbio, C. S. Bayrak, N. Chami, Z. Wang, U. M. Schick, N. Yang, Y. Itan, A. Vidal-Puig,M. den Hoed, S. Mandrup, T. O. Kilpeläinen, R. J. F. Loos (2021) Genome-wide discovery of genetic loci that uncouple excess adiposity from its comorbidities. Nat Metab. 3(2), 228-243.
A. K. Sávári, E. L. Van Hauwaert, L. K. Markussen, E. Gammelmark, A-B. Marcher, M. F. Ebbesen, R. Nielsen, J. R. Brewer, J. G. S. Madsen, S. Mandrup (2021) Plasticity of Edididymal Adipose Tissue in Response to Diet-Induced Obesity at Single-Nucleus Resolution. Cell Metab. 33(2), 437-453.
A.-B. Marcher*, A. Loft*, R. Nielsen, T. Vihervaara, J.G. Madsen, M. Sysi-Aho, K. Ekroos, S. Mandrup (2015) RNA-Seq and Mass-Spectrometry-Based Lipidomics Reveal Extensive Changes of Glycerolipid Pathways in Brown Adipose Tissue in Response to Cold. Cell Reports 13, 2000-2013. (*equal contribution)
A. Loft, I. Forss*, M.S. Siersbæk*, S.F. Schmidt, A.-S. B. Larsen, J.G.S. Madsen, D. Pisani, R. Nielsen, M.M. Aagaard. A. Mathison, M.J. Neville, R. Urrutia, F. Karpe, E.-Z. Amri, S. Mandrup (2015) Browning of human adipocytes requires KLF11 and reprogramming of PPARγ super-enhancers. Genes & Dev. 29, 7-22. (* equal contribution)