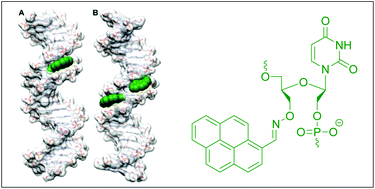
23. Kuhlmann, Matthias; Hamming, Jonas B. R.; Voldum, Anders; Tsakiridou, Georgia; Larsen, Maja T.; Schmoekel, Julie S.; Sohn, Emil; Bienk, Konrad; Schaffert, David; Soerensen, Esben S.; Wengel, Jesper; Dupont, Daniel M.; Howard, Kenneth A. An albumin-oligonucleotide assembly for potential combinatorial drug delivery and half-life extension applications. Molecular Therapy-Nucleic Acids (2017), 9, 284-293. [Abstract]
Abstract: The long blood circulatory property of human serum albumin, due to engagement with the cellular recycling neonatal Fc receptor (FcRn), is an attractive drug half-life extension enabling technol. This work describes a novel site-specific albumin double-stranded (ds) DNA assembly approach, in which the 3' or 5' end maleimide-derivatized oligodeoxynucleotides are conjugated to albumin cysteine at position 34 (cys34) and annealed with complementary strands to allow single site-specific protein modification with functionalized ds oligodeoxynucleotides. Electrophoretic gel shift assays demonstrated successful annealing of complementary strands bearing Atto488, 6-carboxyfluorescein (6-FAM), or a factor IXa aptamer to the albumin-oligodeoxynucleotide conjugate. A fluorometric factor IXa activity assay showed retained aptamer inhibitory activity upon assembly with the albumin and completely blocked factor IXa at a concn. of 100 nM for 2 h. The assembled construct exhibited stability in serum-contg. buffer and FcRn engagement that could be increased using an albumin variant engineered for higher FcRn affinity. This work presents a novel albumin-oligodeoxynucleotide assembly technol. platform that offers potential combinatorial drug delivery and half-life extension applications.
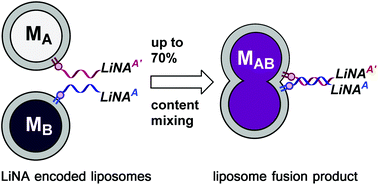
22. Ries, Oliver; Löffler, Philipp M. G.; Rabe, Alexander; Malavan, J. J.; Vogel, Stefan. Efficient liposome fusion mediated by lipid–nucleic acid conjugates. Org. Biomol. Chem. (2017), 15, 8936–8945. [Abstract]
Abstract: The fusion of biomembranes with release of encapsulated content in a controlled way is crucial for cell signaling, endo- and exocytosis and intracellular trafficking. Programmable fusion of liposomes and an efficient mixing of their contents have the potential to enable the study of chemical and enzymatic processes in a confined environment and under crowded conditions outside biological systems. We report on DNA-controlled fusion of lipid bilayer membranes using lipid–nucleic acid conjugates (LiNAs) to mediate lipid and content mixing of liposomes. Screening of different membrane anchor and linker structures as well as incubation temperatures led to significantly improved fusion and content mixing compared to reported systems. LiNA designs were optimized by changing lipophilic moieties as membrane anchors, PEG-spacer patterns and by introducing locked nucleic acid (LNA) modifications. Liposome fusion induced by complementary LiNAs results in remarkable efficient content mixing at 37 °C and 50 °C (up to 70%) with low leakage (≤5%).
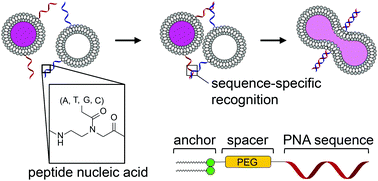
21. Rabe, Alexander; Löffler, Philipp M. G.; Ries, Oliver; Vogel, Stefan. Programmable fusion of liposomes mediated by lipidated PNA. Chem. Commun. (2017), 53, 11921–11924. [Abstract]
Abstract: We recently reported a DNA-programmed fusion cascade enabling the use of liposomes as nanoreactors for compartmentalized chemical reactions. This communication reports an alternative and robust strategy based on lipidated peptide nucleic acids (LiPs). LiPs enabled fusion of liposomes with remarkable 31% efficiency at 50 8C with low leakage (5%).
20. Löffler, Philipp M. G.; Ries, Oliver; Rabe, Alexander; Okholm, Anders H.; Thomsen, Rasmus P.; Kjems, Jørgen; Vogel, Stefan. A DNA-programmed liposome fusion cascade. Angew. Chem. Int. Ed. (2017), 56, 13228–13231. [Abstract]
Abstract: Chemically engineered and functionalized nanoscale compartments are used in bottom-up synthetic biology to construct compartmentalized chemical processes. Progressively more complex designs demand spatial and temporal control over entrapped species. Here, we address this demand with a DNA-encoded design for the successive fusion of multiple liposome populations. Three individual stages of fusion are induced by orthogonally hybridizing sets of membrane-anchored oligonucleotides. Each fusion event leads to efficient content mixing and transfer of the recognition unit for the subsequent stage. In contrast to fusion-protein-dependent eukaryotic vesicle processing, this artificial fusion cascade exploits the versatile encoding potential of DNA hybridization and is generally applicable to small and giant unilamellar vesicles. This platform could thus enable numerous applications in artificial cellular systems and liposome-based synthetic pathways.
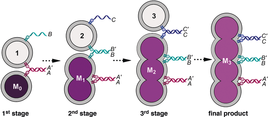
19. Hornum, Mick; Sharma, Pawan K.; Reslow-Jacobsen, Charlotte; Kumar, Pawan; Petersen, Michael; Nielsen, Poul. Condensing the information in DNA with double-headed nucleotides. Chemical Communications (2017), 53(70), 9717-9720. [Abstract]
Abstract: A normal duplex holds as many Watson–Crick base pairs as the number of nucleotides in its constituent strands. Here we establish that single nucleotides can be designed to functionally imitate dinucleotides without compromising binding affinity. This effectively allows sequence information to be more compact and concentrated to fewer phosphates.

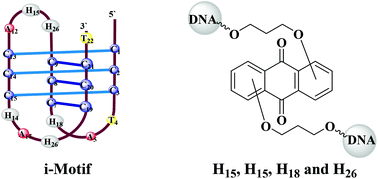
18. Gouda, Alaa S.; Amine, Mahasen S.; Pedersen, Erik B. Improved i-motif thermal stability by insertion of anthraquinone monomers. Organic & Biomolecular Chemistry (2017), 15, 6613-6621. [Abstract].
Abstract: In order to gain insight into how to improve thermal stability of i-motifs when used in the context of biomedical and nanotechnological applications, novel anthraquinone-modified i-motifs were synthesized by insertion of 1,8-, 1,4-, 1,5- and 2,6-disubstituted anthraquinone monomers into the TAA loops of a 22mer cytosine-rich human telomeric DNA sequence. The influence of the four anthraquinone linkers on the i-motif thermal stability was investigated at 295 nm and pH 5.5. Anthraquinone monomers modulate the i-motif stability in a position-depending manner and the modulation also depends on the substitution pattern of the anthraquinone. The insertion of anthraquinone was found to stabilize the i-motif structure when replacing any one of the positions of the central TAA loop and the thermal stabilities were typically higher than those previously found for i-motifs containing pyrene-modified uracilyl unlocked nucleic acid monomers or twisted intercalating nucleic acid. The 2,6-disubstituted anthraquinone linker replacing T10 enabled a significant increase of i-motif thermal melting by 8.2 °C. A substantial increase of 5.0 °C in i-motif thermal melting was recorded when both A6 and T16 were modified with a double replacement by the 2,6-isomer into the TAA loops in the outer regions. The largest destabilization is observed for the 1,5-disubstituted anthraquinone linker upon the replacement of A18. CD curves of anthraquinone-modified variants imply no structural changes in all cases under potassium buffer conditions compared with those of the native i-motif. Molecular modeling studies explained the increased thermal stabilities of anthraquinone-modified i-motifs.
17. Taskova, Maria; Barducci, M. C.; Astakhova, Kira. Environmentally sensitive molecular probes reveal mutations and epigenetic 5-methyl cytosine in human oncogenes. Organic & Biomolecular Chemistry (2017),15, 5680-5684. [Abstract].
Abstract: There is currently an unmet need for reliable tools that allow for direct detection and quantification of modifications in genomic DNA. For example, in cancer research and clinical diagnostics, target DNA has to be amplified and sequenced in order to reveal mutations. For 5-methylcytosine detection, bisulfite treatment of DNA is applied for the analysis, which often leads to poor specificity and reproducibility of the results. Herein we describe a simple approach that specifically detects clinically significant modifications in the human oncogenes BRAF and KRAS. We prove that this can be done using a fast and reliable hybridization assay applying novel internally labelled oligonucleotide probes and optical detection methods.

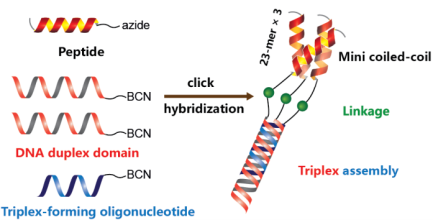
16. Lou, Chenguang; Christensen, Niels Johan; Martos-Maldonado, Manuel C.; Midtgaard, Soren Roi; Ejlersen, Maria; Thulstrup, Peter W.; Sorensen, Kasper K.; Jensen, Knud J.; Wengel, Jesper. Folding topology of a short coiled-coil peptide structure templated by an oligonucleotide triplex. Chemistry - A European Journal (2017), 23(39), 9297-9305. (Hot Paper) [Abstract] [Inside Cover Picture]
Abstract: The rational design of a well-defined protein-like tertiary structure formed by small peptide building blocks is still a formidable challenge. By using peptide–oligonucleotide conjugates (POC) as building blocks, we present the self-assembly of miniature coiled-coil α-helical peptides guided by oligonucleotide duplex and triplex formation. POC synthesis was achieved by copper-free alkyne–azide cycloaddition between three oligonucleotides and a 23-mer peptide, which by itself exhibited multiple oligomeric states in solution. The oligonucleotide domain was designed to furnish a stable parallel triplex under physiological pH, and to be capable of templating the three peptide sequences to constitute a small coiled-coil motif displaying remarkable α-helicity. The formed trimeric complex was characterized by ultraviolet thermal denaturation, gel electrophoresis, circular dichroism (CD) spectroscopy, small-angle X-ray scattering (SAXS), and molecular modeling. Stabilizing cooperativity was observed between the trimeric peptide and the oligonucleotide triplex domains, and the overall molecular size (ca. 12 nm) in solution was revealed to be independent of concentration. The topological folding of the peptide moiety differed strongly from those of the individual POC strands and the unconjugated peptide, exclusively adopting the designed triple helical structure.
15. Hvam, Michael Lykke; Cai, Yunpeng; Dagnaes-Hansen, Frederik; Nielsen, Jesper Sejrup; Wengel, Jesper; Kjems, Jørgen; Howard, Kenneth A. Fatty acid-modified gapmer antisense oligonucleotide and serum albumin constructs for pharmacokinetic modulation. Molecular Therapy (2017), 25(7), 1710-1717. [Abstract].
Abstract: Delivery technologies are required for realizing the clinical potential of molecular medicines. This work presents an alternative technology to preformulated delivery systems by harnessing the natural transport properties of serum albumin using endogenous binding of gapmer antisense oligonucleotides (ASOs)/albumin constructs. We show by an electrophoretic mobility assay that fatty acid-modified gapmer and human serum albumin (HSA) can self-assemble into constructs that offer favorable pharmacokinetics. The interaction was dependent on fatty acid type (either palmitic or myristic acid), number and position within the gapmer ASO sequence, as well as phosphorothioate (PS) backbone modifications. Binding correlated with increased blood circulation in mice (t1/2 increased from 23 to 49 min for phosphodiester [PO] gapmer ASOs and from 28 to 66 min for PS gapmer ASOs with 2× palmitic acid modification). Furthermore, a shift toward a broader biodistribution was detected for PS compared with PO gapmer ASOs. Inclusion of 2× palmitoyl to the ASOs shifted the biodistribution to resemble that of natural albumin. This work, therefore, presents a novel strategy based on the proposed endogenous assembly of gapmer ASOs/albumin constructs for increased circulatory half-life and modulation of the biodistribution of gapmer ASOs that offers tunable pharmacokinetics based on the gapmer modification design.
14. Gouda, Alaa S.; Amine, Mahasen S.; Pedersen, Erik B. Synthesis and molecular modeling of thermally stable DNA G-quadruplexes with anthraquinone insertions. European Journal of Organic Chemistry (2017), 2017(21), 3092-3100. [Abstract].
Abstract: Two new phosphoramidite building blocks for DNA synthesis were synthesized from 1,5- and 2,6-dihydroxyanthraquinones through alkylation with 3-bromo-1-propanol followed by DMT-protection. The novel synthesized 1,5- and 2,6-disubstituted anthraquinone monomers H15 and H26 are incorporated into a G-quadruplex by single and double replacements of TGT and TT loops. Monomers H15 and H26 were found to destabilize G-quadruplex structures for all single replacements of TGT or TT loops. The largest destabilization was obsd. when H26 linker replaced a TT loop. In contrast, the presence of anthraquinone monomers in two TT loops led to 1-18 °C increase in their thermal stabilities, depending on linker attachment geometry of the monomers. The presence of H15 and H26 linkers replacing two TT loops results in the highest stabilization of the G-quadruplex structure by 18.2 °C. CD spectroscopy of all anthraquinone-modified quadruplexes revealed no change of the antiparallel structure when compared with the wild type under potassium buffer conditions. The significantly increased thermostabilities were interpreted by mol. modeling of anthraquinone-modified G-quadruplexes.
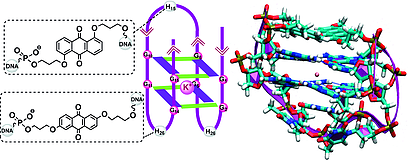
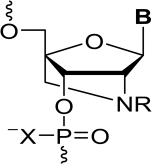
13. Vejlegaard, Kim; Paul, Sibasish; Kosbar, Tamer; Wengel, Jesper; Caruthers, Marvin H. Oligodeoxynucleotides containing 2'-amino-LNA nucleotides as constrained morpholino phosphoramidate and phosphorodiamidate monomers. Bioorganic and Medicinal Chemistry Letters (2017), 27(14), 3173-3176. [Abstract].
Abstract: Incorporation in a 2' → 5' direction of a phosphorodiamidite 2'-amino-LNA-T nucleotide as the morpholino phosphoramidate and N,N-dimethylamino phosphorodiamidate monomers into six oligonucleotides is reported. Thermal denaturation studies showed that the novel 2'-amino-LNA-based morpholino monomers exert a destabilizing effects on duplexes formed with complementary DNA and RNA.
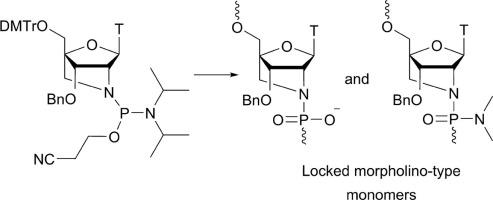
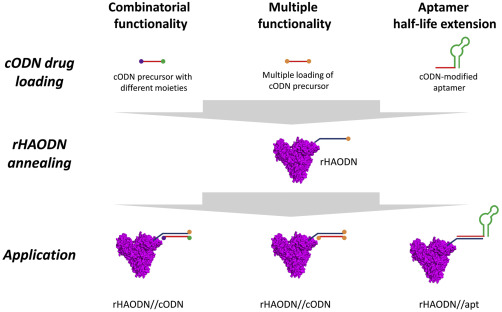
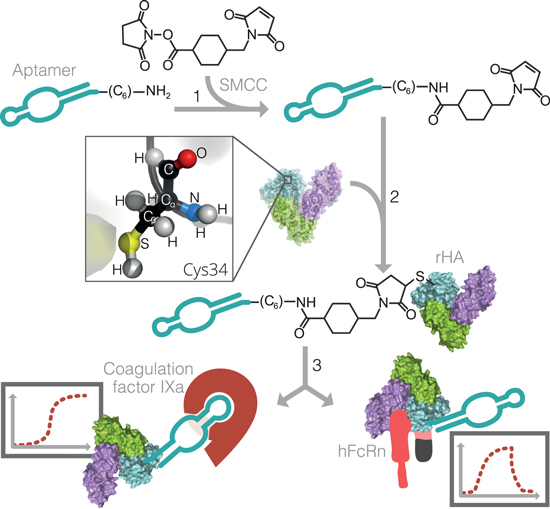
12. Schmokel, Julie; Voldum, Anders; Tsakiridou, Georgia; Kuhlmann, Matthias; Cameron, Jason; Sørensen, Esben S; Wengel, Jesper; Howard, Kenneth A. Site-selective conjugation of an anticoagulant aptamer to recombinant albumins and maintenance of neonatal Fc receptor binding. Nanotechnology (2017), 28(20), 204004. [Abstract].
Abstract: Aptamers are an attractive molecular medicine that offers high target specificity. Nucleic acid-based aptamers, however, are prone to nuclease degradation and rapid renal excretion that require blood circulatory half-life extension enabling technologies. The long circulatory half-life, predominately facilitated by engagement with the cellular recycling neonatal Fc receptor (FcRn), and ligand transport properties of albumin promote it as an attractive candidate to improve the pharmacokinetic profile of aptamers. This study investigates the effect of Cys34 site-selective covalent attachment of a factor IXa anticoagulant aptamer on aptamer functionality and human FcRn (hFcRn) engagement using recombinant human albumin (rHA) of either a wild type (WT) or an engineered human FcRn high binding variant (HB). Albumin-aptamer conjugates, connected covalently through a heterobifunctional succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate linker, were successfully prepared and purified by high performance liquid chromatography as confirmed by gel electrophoresis band-shift analysis and matrix-assisted laser desorption/ionization time of flight. Minimal reduction (∼25%) in activity of WT-linked aptamer to that of aptamer alone was found using an anticoagulant activity assay measuring temporal levels of activated partial thrombin. Covalent albumin-aptamer conjugation, however, substantially compromized binding to hFcRn, to 10% affinity of that of non-conjugated WT, determined by biolayer interferometry. Binding could be rescued by aptamer conjugation to recombinant albumin engineered for higher FcRn affinity (HB) that exhibited an 8-fold affinity compared to WT alone. This work describes a novel albumin-based aptamer delivery system whose hFcRn binding can be increased using a HB engineered albumin.
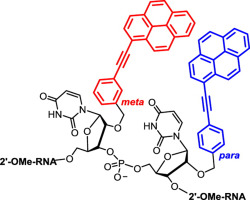
11. Astakhova, Kira; Golovin, Andrey V.; Prokhorenko, Igor A.; Ustinov, Alexey V.; Stepanova, Irina A.; Zatsepin, Timofei S.; Korshun, Vladimir A. Design of 2'-phenylethynylpyrene excimer forming DNA/RNA probes for homogeneous SNP detection: The attachment manner matters. Tetrahedron (2017), 73(23), 3220-3230. [Abstract]
Abstract: 1-Phenylethynylpyrene fluorophore (1-PEPy) has long-wavelength shifted emission and higher photostability compared to pyrene, retaining, however, pyrene's ability to form excimers. Here we report the synthesis of 2′-O-[3(and 4)-(pyren-1-ylethynyl)benzyl]-uridines and their tandem incorporation into deoxyribo- and 2′-O-Me-ribo-oligonucleotide probes. Excimer forming probes of type NN … NNXXNN … NN (X = 2′-O-[meta(or para)-(pyren-1-ylethynyl)-benzyl]uridine) containing two adjacent fluorescent nucleosides within an oligonucleotide are available in four types (meta-meta; para-meta; meta-para; para-para). Both DNA (N = deoxyribonucleotides) and 2′-O-Me-RNA (N = 2′-O-Me-ribo-nucleotides) probes were synthesized and their hybridization with complementary and singly mismatched DNA and RNA was studied. Several probes show a dramatic response of their excimer-to-monomer intensity ratio upon hybridization. Remarkably, most spectacular fluorescence changes were demonstrated for probes with para-meta and meta-para combination within 2′-O-Me-ribo-oligonucleotides. Using excimer forming probes, three natural SNP in Helicobacter pylori 23S RNA gene (A2144G, A2143G, A2143C) and the wild type gene can be distinguished.
10. Hartono, Yossa Dwi; Pabon-Martinez, Y. Vladimir; Uyar, Arzu; Wengel, Jesper; Lundin, Karin E.; Zain, Rula; Smith, C. I. Edvard; Nilsson, Lennart; Villa, Alessandra. Role of pseudoisocytidine tautomerization in triplex-forming oligonucleotides: In silico and in vitro studies. ACS Omega (2017), 2(5), 2165-2177. [Abstract]
Abstract: Pseudoisocytidine (ΨC) is a synthetic cytidine analog that can target DNA duplex to form parallel triplex at neutral pH. Pseudoisocytidine has mainly two tautomers, of which only one is favorable for triplex formation. In this study, we investigated the effect of sequence on Ψ tautomerization using λ-dynamics simulation, which takes into account transitions between states. We also performed in vitro binding expts. with sequences contg. ΨC and, furthermore, characterized the structure of the formed triplex with mol. dynamics simulation. We have found that neighboring methylated or protonated cytidine promotes the formation of the favorable tautomer, while neighboring thymine or locked nucleic acid (LNA) has poor effect, and consecutive ΨC has a neg. influence. The deleterious effect of consecutive ΨC in triplex formation is confirmed using in vitro binding expts. Our findings contribute to improve the design of ΨC-contg. triplex-forming oligonucleotides directed to target G-rich DNA sequences.
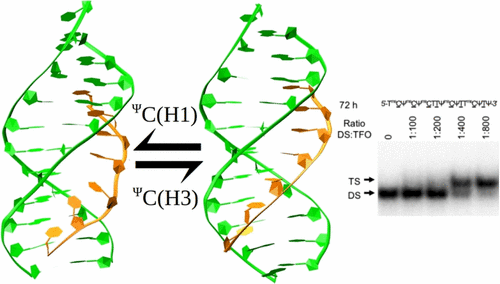
9. Zaghloul, Eman M; Gissberg, Olof; Moreno, Pedro M D; Siggens, Lee; Hällbrink, Mattias, Jørgensen, Anna S; Ekwall, Karl; Zain, Rula; Wengel, Jesper; Lundin, Karin E; Smith, C I Edvard. CTG repeat-targeting oligonucleotides for down-regulating Huntingtin expression. Nucleic acids research (2017), 45(9), 5153-5169. [Open Access].
Abstract: Huntington's disease (HD) is a fatal, neurodegenerative disorder in which patients suffer from mobility, psychological and cognitive impairments. Existing therapeutics are only symptomatic and do not significantly alter the disease progression or increase life expectancy. HD is caused by expansion of the CAG trinucleotide repeat region in exon 1 of the Huntingtin gene (HTT), leading to the formation of mutant HTT transcripts (muHTT). The toxic gain-of-function of muHTT protein is a major cause of the disease. In addition, it has been suggested that the muHTT transcript contributes to the toxicity. Thus, reduction of both muHTT mRNA and protein levels would ideally be the most useful therapeutic option. We herein present a novel strategy for HD treatment using oligonucleotides (ONs) directly targeting the HTT trinucleotide repeat DNA. A partial, but significant and potentially long-term, HTT knock-down of both mRNA and protein was successfully achieved. Diminished phosphorylation of HTT gene-associated RNA-polymerase II is demonstrated, suggestive of reduced transcription downstream the ON-targeted repeat. Different backbone chemistries were found to have a strong impact on the ON efficiency. We also successfully use different delivery vehicles as well as naked uptake of the ONs, demonstrating versatility and possibly providing insights for in vivo applications.
8. Kumar, Rajesh; Ries, Annika; Wengel, Jesper. Synthesis and excellent duplex stability of oligonucleotides containing 2′-amino-LNA functionalized with galactose units. Molecules (2017), 22(5), 852. [Open Access].
Abstract: A convenient method for the preparation of oligonucleotides containing internally-attached galactose and triantennary galactose units has been developed based on click chemistry between 2′-N-alkyne 2′-amino-LNA nucleosides and azido-functionalized galactosyl building blocks. The synthesized oligonucleotides show excellent binding affinity and selectivity towards complementary DNA/RNA strands with an increase in the melting temperature of up to +23.5 °C for triply-modified variants.
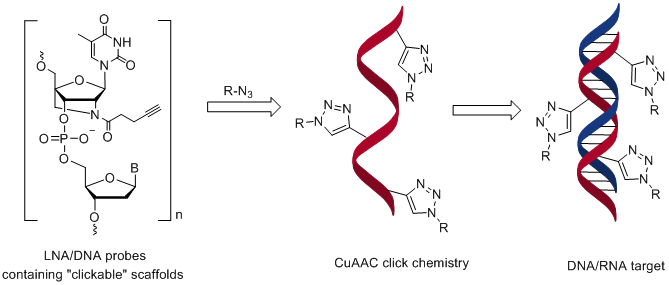
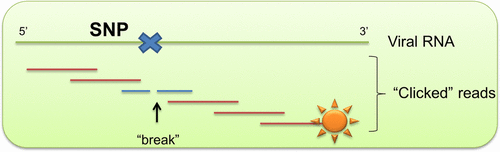
7. Taskova, Maria; Uhd, Jesper; Miotke, Laura; Kubit, Matthew; Bell, John; Ji, Hanlee P.; Astakhova, Kira. Tandem oligonucleotide probe annealing and elongation to discriminate viral sequence. Analytical Chemistry, (2017), 89(8), 4363-4366. [Abstract].
Abstract: New approaches for genomic DNA/RNA detection are in high demand in order to provide controls for existing enzymic technologies and to create alternatives for emerging applications. In particular, there is an unmet need in rapid, reliable detection of short RNA regions which could open up new opportunities in transcriptome anal., virol., and other fields. Herein, the authors report for the first time a "click" chem. approach to oligonucleotide probe elongation as a novel approach to specifically detect a viral sequence. The authors hybridized a library of short, terminally labeled probes to Ebola virus RNA followed by click assembly and anal. of the read sequence by various techniques. As the authors demonstrate in this paper, using the new approach, a viral RNA sequence can be detected in less than 2 h without the need for cDNA synthesis or any other enzymic reactions and with a sensitivity of <10 pM target RNA.
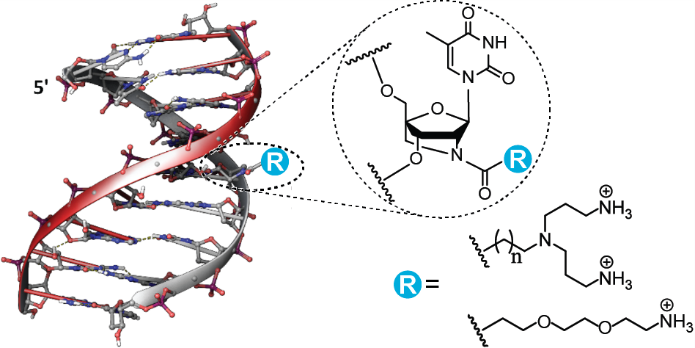
6. Lou, Chenguang; Samuelsen, Simone V.; Christensen, Niels Johan; Vester, Birte; Wengel, Jesper. Oligonucleotides Containing Aminated 2'-Amino-LNA Nucleotides: Synthesis and Strong Binding to Complementary DNA and RNA. Bioconjugate Chemistry (2017), 28(4), 1214-1220. [Abstract].
Abstract: Mono- and diaminated 2′-amino-LNA monomers were synthesized and introduced into oligonucleotides. Each modification imparts significant stabilization of nucleic acid duplexes and triplexes, excellent sequence selectivity, and significant nuclease resistance. Molecular modeling suggested that structural stabilization occurs via intrastrand electrostatic attraction between the protonated amino groups of the aminated 2′-amino-LNA monomers and the host oligonucleotide backbone.
5. Aaldering, Lukas J.; Poongavanam, Vasanthanathan; Langkjær, Niels; Murugan, N. Arul; Jørgensen, Per Trolle; Wengel, Jesper; Veedu, Rakesh N. Development of an efficient G-quadruplex-stabilised Thrombin-binding aptamer containing a three-carbon spacer molecule. ChemBioChem (2017), 18, 755– 763. [Abstract].
Abstract: The thrombin-binding aptamer (TBA), which shows anticoagulant properties, is one of the most studied G-quadruplex-forming aptamers. In this study, we investigated the impact of different chemical modifications such as a three-carbon spacer (spacer-C3), unlocked nucleic acid (UNA) and 3′-amino-modified UNA (amino-UNA) on the structural dynamics and stability of TBA. All three modifications were incorporated at three different loop positions (T3, T7, T12) of the TBA G-quadruplex structure to result in a series of TBA variants and their stability was studied by thermal denaturation; folding was studied by circular dichroism spectroscopy and thrombin clotting time. The results showed that spacer-C3 introduction at the T7 loop position (TBA-SP7) significantly improved stability and thrombin clotting time while maintaining a similar binding affinity as TBA to thrombin. Detailed molecular modelling experiments provided novel insights into the experimental observations, further supporting the efficacy of TBA-SP7. The results of this study could provide valuable information for future designs of TBA analogues with superior thrombin inhibition properties.
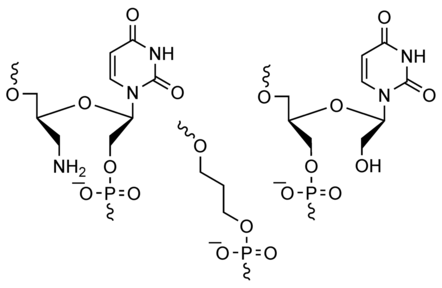
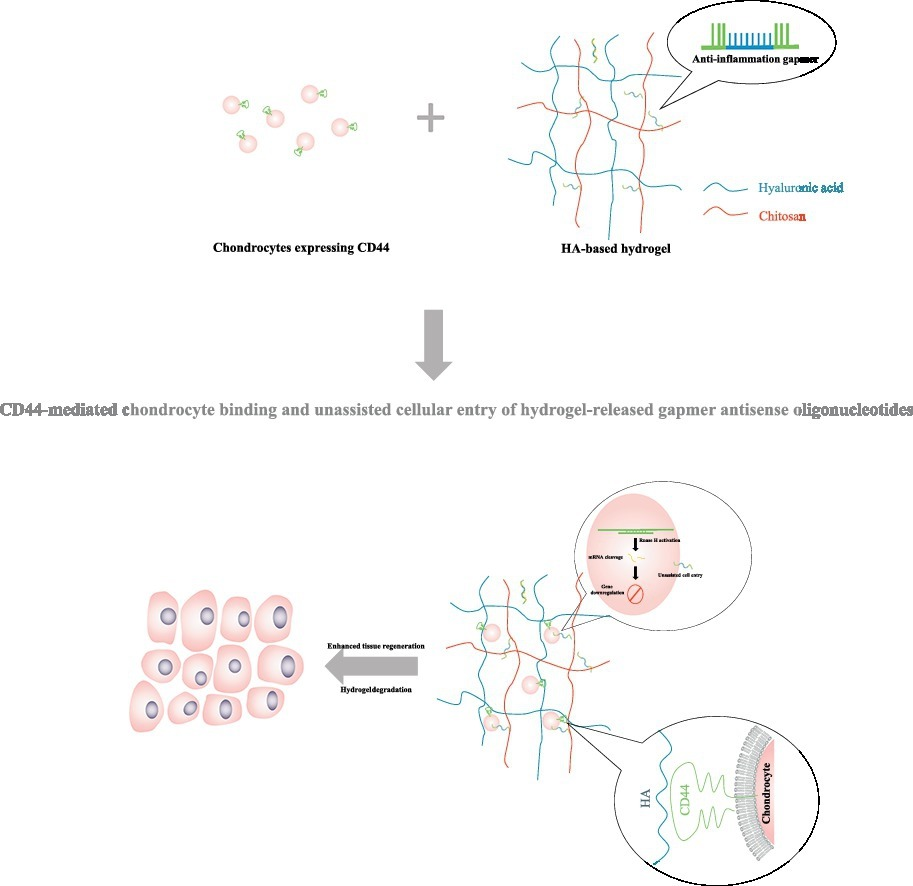
4. Cai, Yunpeng; Lopez-Ruiz, Elena; Wengel, Jesper; Creemers, Laura B.; Howard, Kenneth A. A hyaluronic acid-based hydrogel enabling CD44-mediated chondrocyte binding and gapmer oligonucleotide release for modulation of gene expression in osteoarthritis. Journal of Controlled Release (2017), 53, 153-159 [Abstract].
Abstract: Hyaluronic acid (HA) is an attractive biomaterial for osteoarthritis (OA) treatment due to inherent functional and compatibility properties as an endogenous knee joint component. In this work, we describe a HA-based hydrogel with the dual functionality of increased CD44-dependent chondrocyte binding and controlled release of gapmer antisense oligonucleotides for unassisted cellular entry and subsequent gene silencing activity. A Schiff base-mediated gelation method was used to produce a panel of hydrogels varying in the aldehyde-modified HA (900 kDa) to chitosan ratios (3:7, 5:5 and 7:3) for identifying designs displaying optimal engagement of OA patient-derived CD44-expressing chondrocytes. Correlation was found between cell binding and CD44 expression, with maximal binding exhibited at a HA/chitosan ratio of 7:3, that was 181% higher than CD44-negative MCF-7 cell control cells. Transfection agent-free uptake into OA chondrocytes of fluorescent 13-mer DNA oligonucleotides with a flanked locked nucleic acid (LNA) gapmer design, in contrast to naked siRNA, was demonstrated by confocal and flow cytometric analysis. A sustained and complete release over 5 days was found with the 7:3 hydrogel, in contrast, the 5:5 and 3:7 hydrogel released 60% and 43% of loaded gapmers, respectively over the same period. A COX-2-specific gapmer designed with maximal chondrocyte gene silencing (~ 70% silencing efficiency at 500 nM compared with a mismatch gapmer sequence) resulted in effective COX-2 silencing over 14 days in hydrogels seeded with OA chondrocytes, with significant difference exhibited between day 3 and 10. This work introduces a novel HA-based CD44-mediated cellular binding and gapmer controlled release platform to modulate cellular gene expression.
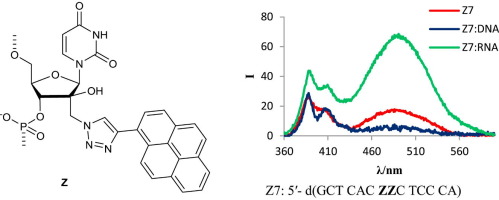
3. Kumar, Pawan; Sharma, Pawan K.; Nielsen, Poul; Synthesis, hybridization and fluorescence properties of a 2'-C-pyrene-triazole modified arabino-uridine nucleotide. Bioorganic & Medicinal Chemistry (2017), 25(7), 2084-2090 [Abstract].
Abstract: A new pyrene-modified nucleotide monomer is introduced, wherein pyrene is attached to the 20-position of arabino-uridine through a triazolemethyl linker. When the monomer is introduced into oligonucleotides, very stable DNA duplexes and three way junctions are obtained. An oligonucleotide featuring two modifications in the center shows four-fold increase in the intensity of the pyrene excimer signal on hybridization with an RNA target but not with a DNA target. The new nucleotide monomer has potential in DNA invader probes as well as in RNA targeting and detection.
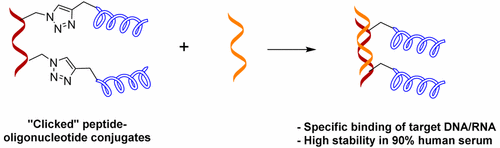
2. Taskova, Maria; Madsen, Charlotte S.; Jensen, Knud J.; Hansen, Lykke Haastrup; Vester, Birte; Astakhova, Kira. Antisense oligonucleotides internally labeled with peptides show improved target recognition and stability to enzymatic degradation. Bioconjugate Chemistry (2017), 28(3), 768-774 [Abstract].
Abstract: Specific target binding and stability in diverse biological media is of crucial importance for applications of synthetic oligonucleotides as diagnostic and therapeutic tools. So far, these issues have been addressed by chemical modification of oligonucleotides and by conjugation with a peptide, most often at the terminal position of the oligonucleotide. Herein, we for the first time systematically investigate the influence of internally attached short peptides on the properties of antisense oligonucleotides. We report the synthesis and internal double labeling of 21-mer oligonucleotides that target the BRAF V600E oncogene, with a library of rationally designed peptides employing CuAAC “click” chemistry. The peptide sequence has an influence on the specificity and affinity of target DNA/RNA binding. We also investigated the impact of locked nucleic acids (LNAs) on the latter. Lysine residues improve binding of POCs to target DNA and RNA, whereas the distance to lysine correlates exclusively with a decrease in binding of mismatched RNA targets. Glycine and tyrosine residues affect target binding as well. Importantly, the resistance of POCs to enzymatic degradation is dramatically improved by the internal attachment of peptides but not by LNA alone. Independently of the peptide sequence, the conjugates are stable for up to 24 h in 90% human serum and duplexes of POCs with complementary DNA for up to 160 h in 90% human serum. Such excellent stability has not been previously reported for DNA and makes internally labeled POCs an exciting object of study, i.e., showing high target specificity and simultaneous stability in biological media.
1. Ejlersen, Maria; Langkjaer, Niels; Wengel, Jesper. 3'-Pyrene-modified unlocked nucleic acids: synthesis, fluorescence properties and a surprising stabilization effect on duplexes and triplexes. Organic & Biomolecular Chemistry (2017), 15, 2073–2085 [Abstract].
Abstract: Efficient synthesis of a new 3'-O-amino-UNA monomer was developed as a scaffold for further functionalization and incorporation into oligonucleotides (ONs). Pyrene-functionalized 3'-O-amino-UNA was incorporated one, two or three times into 21-mer DNA and 2'-O-Me-RNA ONs. Duplex melting temps., CD (CD) spectra, steady-state fluorescence emission spectra, UV/Vis absorption spectra and triplex melting temps. were measured for the modified duplexes. The presence of the pyrene-modified UNA monomer lead to a surprising and unprecedented thermal stabilization of esp. DNA:DNA duplexes when compared to the corresponding unmodified DNA:DNA duplexes. Improved mismatch discrimination was also seen for some of the modified duplexes. CD spectra revealed no major differences between modified and unmodified duplexes. Mol. modeling showed that the pyrene moieties were located in the minor groove of DNA:DNA duplexes as confirmed by CD and UV/Vis absorption studies. Upon multiple incorporations of the monomer in single-stranded ONs, steady-state fluorescence emission studies revealed the formation of a pyrene excimer which in most cases was quenched upon duplex hybridization, and fluorescence-based detection of mismatched hybridization was obsd. for some modified strand constitutions. Incorporation of the monomer in a triplex-forming oligonucleotide (TFO) strand lead to an increase of triplex melting temp. both at pH 6.0 and pH 7.0 for parallel triplexes - again an effect that has not been reported earlier for UNA-contg. ONs. Steady-state fluorescence emission studies revealed significant differences in fluorescence for single-stranded ONs and triplexes.