17. Cogoi, S, Jakobsen, U, Pedersen, EB, Vogel, S & Xodo, LE. Lipid-modified G4-decoy oligonucleotide anchored to nanoparticles: delivery and bioactivity in pancreatic cancer cells. Scientific Reports, (2016), 6, 38468. [Open Access]
Abstract: KRAS is mutated in >90% of pancreatic ductal adenocarcinomas. As its inactivation leads to tumour regression, mutant KRAS is considered an attractive target for anticancer drugs. In this study we report a new delivery strategy for a G4-decoy oligonucleotide that sequesters MAZ, a transcription factor essential for KRAS transcription. It is based on the use of palmitoyl-oleyl-phosphatidylcholine (POPC) liposomes functionalized with lipid-modified G4-decoy oligonucleotides and a lipid-modified cell penetrating TAT peptide. The potency of the strategy in pancreatic cancer cells is demonstrated by cell cytometry, confocal microscopy, clonogenic and qRT-PCR assays.
16. Ries, Annika; Kumar, Rajesh; Lou, Chenguang; Kosbar, Tamer; Vengut-Climent, Empar; Jørgensen, Per T.; Morales, Juan C.; Wengel, Jesper. Synthesis and biophysical investigations of oligonucleotides containing galactose-modified DNA, LNA, and 2'-amino-LNA monomers. Journal of Organic Chemistry (2016), 81(22), 10845-10856. [Abstract]
Abstract: Galactose-modified thymidine, LNA-T, and 2′-amino-LNA-T nucleosides were synthesized, converted into the corresponding phosphoramidite derivatives and introduced into short oligonucleotides. Compared to the unmodified control strands, the galactose-modified oligonucleotides in general, and the N2′-functionalized 2′-amino-LNA derivatives in particular, showed improved duplex thermal stability against DNA and RNA complements and increased ability to discriminate mismatches. In addition, the 2′-amino-LNA-T derivatives induced remarkable 3′-exonuclease resistance. These results were further investigated using molecular modeling studies.
15. Junager Nina, P.L; Kongsted Jakob; Astakhova Kira.: Revealing nucleic acid mutations using Förster resonance energy transfer-based probes. Sensors (2016),16(8). 1173. [Open Access]
Abstract: Nucleic acid mutations are of tremendous importance in modern clinical work, biotechnology and in fundamental studies of nucleic acids. Therefore, rapid, cost-effective and reliable detection of mutations is an object of extensive research. Today, Förster resonance energy transfer (FRET) probes are among the most often used tools for the detection of nucleic acids and in particular, for the detection of mutations. However, multiple parameters must be taken into account in order to create efficient FRET probes that are sensitive to nucleic acid mutations. In this review; we focus on the design principles for such probes and available computational methods that allow for their rational design. Applications of advanced, rationally designed FRET probes range from new insights into cellular heterogeneity to gaining new knowledge of nucleic acid structures directly in living cells.
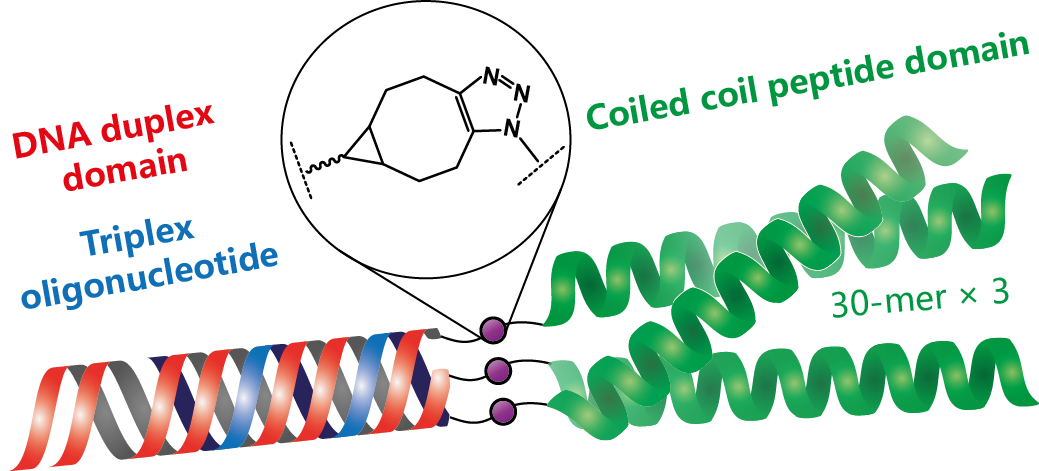
14. Lou Chenguang; Martos-Maldonado Manuel C; Madsen Charlotte S; Thomsen Rasmus P; Midtgaard Søren Roi; Christensen Niels Johan; Kjems Jørgen; Thulstrup Peter W; Wengel Jesper; Jensen Knud J.: Peptide-oligonucleotide conjugates as nanoscale building blocks for assembly of an artificial three-helix protein mimic. Nature Communications (2016), 7, 12294. [Abstract]
Abstract: Peptide-based structures can be designed to yield artificial proteins with specific folding patterns and functions. Template-based assembly of peptide units is one design option, but the use of two orthogonal self-assembly principles, oligonucleotide triple helix and a coiled coil protein domain formation have never been realized for de novo protein design. Here, we show the applicability of peptide–oligonucleotide conjugates for self-assembly of higher-ordered protein-like structures. The resulting nano-assemblies were characterized by ultraviolet-melting, gel electrophoresis, circular dichroism (CD) spectroscopy, small-angle X-ray scattering and transmission electron microscopy. These studies revealed the formation of the desired triple helix and coiled coil domains at low concentrations, while a dimer of trimers was dominating at high concentration. CD spectroscopy showed an extraordinarily high degree of α-helicity for the peptide moieties in the assemblies. The results validate the use of orthogonal self-assembly principles as a paradigm for de novo protein design.
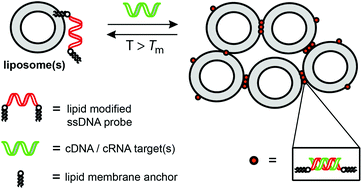
13. Jakobsen, Ulla; Vogel, Stefan.: Mismatch discrimination of lipidated DNA and LNA-probes (LiNAs) in hybridization-controlled liposome assembly. Organic & Biomolecular Chemistry (2016), 14(29), 6985-6995. [Abstract]
Abstract: Assays for mismatch discrimination and detection of single nucleotide variations by hybridization-controlled assembly of liposomes, which do not require tedious surface chem., are versatile for both DNA and RNA targets. We report herein a comprehensive study on different DNA and LNA (locked nucleic acids) probe designs, including membrane-anchoring requirements, studies on different probes and target lengths (including overhangs), DNA and RNA targets (including sequences assocd. with pathogens) for lipidated nucleic acids (LiNAs). Advantages and limitations of the liposome assembly based assay in the context of mismatch discrimination and SNP detection are presented. The advantages of membrane-anchored LiNA-probes compared to chem. attached probes on solid nanoparticles (e.g. gold nanoparticles) are described. Key functionalities such as non-covalent attachment of LiNA probes without the need for long spacers and the inherent mobility of membrane-anchored probes in lipid-bilayer membranes will be described for several different probe designs.
12. Samuelsen Simone V; Maity Arindam; Astakhova Kira; Nybo Mads; Macaubas Claudia; Balboni Imelda M; Mellins Elizabeth D; Lonstrup Lars. Novel phospholipid-protein conjugates allow improved detection of antibodies in patients with autoimmune diseases. PloS one (2016), 11(6), e0156125. [Open Access]
Abstract: Reliable measurement of clinically relevant autoimmune antibodies toward phospholipid-protein conjugates is highly desirable in research and clinical assays. To date, the development in this field has been limited to the use of natural heterogeneous antigens. However, this approach does not take structural features of biologically active antigens into account and leads to low reliability and poor scientific test value. Here we describe novel phospholipid-protein conjugates for specific detection of human autoimmune antibodies. Our synthetic approach includes mild oxidation of synthetic phospholipid cardiolipin, and as the last step, coupling of the product with azide-containing linker and copper-catalyzed click chemistry with β2-glycoprotein I and prothrombin. To prove utility of the product antigens, we used enzyme-linked immunosorbent assay and three cohorts of samples obtained from patients in Denmark (n = 34) and the USA (n = 27 and n = 14). Afterwards we analyzed correlation of the obtained autoantibody titers with clinical parameters for each patient. Our results prove that using novel antigens clinically relevant autoantibodies can be detected with high repeatability, sensitivity and specificity. Unlike previously used antigens the obtained autoantibody titers strongly correlate with high disease activity and in particular, with arthritis, renal involvement, anti-Smith antibodies and high lymphocyte count. Importantly, chemical composition of antigens has a strong influence on the correlation of detected autoantibodies with disease activity and manifestations. This confirms the crucial importance of antigens’ composition on research and diagnostic assays, and opens up exciting perspectives for synthetic antigens in future studies of autoimmunity.
11. Gissberg Olof; Zaghloul Eman M; Lundin Karin E; Zain Rula; Smith C I Edvard; Nguyen Chi-Hung; Landras-Guetta Corinne; Wengel Jesper; Zain Rula. Delivery, effect on cell viability, and plasticity of modified aptamer constructs. Nucleic acid therapeutics (2016), 26 (3), 183-189. [Abstract]
Abstract: AS1411 is a G-quadruplex-forming aptamer capable of selectively entering cancer cells by nucleolin receptor-mediated uptake. In this study, we investigated the cell internalization properties and plasticity of AS1411 carrying different locked nucleic acid-containing cargo oligonucleotides (ONs) for delivery into A549 and U2OS cells. We found that internalization efficiency is highly governed by ON cargo chemistry and composition since the inherent antitumor properties of AS1411 were lost when attached to a nontoxic ON, noTox. However, a toxic ON, Tox, demonstrated potent cytotoxicity after aptamer-mediated uptake in A549 cells. We also examined the effect of unlocked nucleic acid (UNA) modifications in the loop region of the aptamer, and how the cargo ONs and UNA incorporation affect the secondary structure of AS1411, in the presence or absence of two novel ellipticine derivatives. These findings add new insights to the design and future applications of aptamer-guided delivery of ON cargo to cancer cells.
10. Hornum, Mick; Djukina, Alevtina; Sassnau, Ann-Katrin; Nielsen, Poul. Synthesis of new C-5-triazolyl-functionalized thymidine analogs and their ability to engage in aromatic stacking in DNA : DNA and DNA : RNA duplexes. Organic & Biomolecular Chemistry (2016), 14 (19), 4436-4447. [Abstract]
Abstract: 1-Phenyl-1,2,3-triazole scaffolds on the 5-position of pyrimidine nucleosides have previously shown to enhance nuclease stability and increase the duplex thermal stability (Tm) by engaging in duplex stacking interactions. In this study, we have introduced two new derivatives of this scaffold in DNA:DNA and DNA:RNA duplexes in order to explore the thermal effects of (1) using a 1,5-triazole instead of the usual 1,4-triazole, and (2) replacing the apolar phenyl substituent with a polar uracil-5-yl substituent.

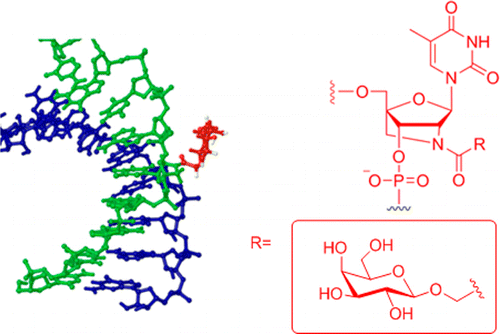
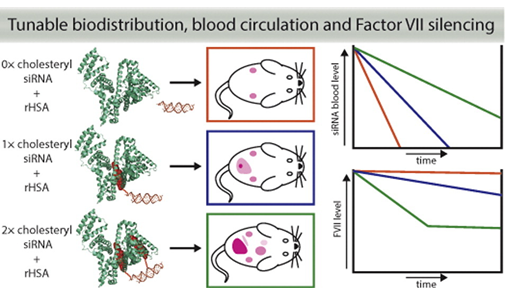
9. Bienk Konrad; Hvam Michael Lykke; Pakula Malgorzata Maria; Dagn¡áús-Hansen Frederik; Kragh-Hansen Ulrich; Wengel Jesper; Malle Birgitte Molholm; Bukrinski Jens Thostrup; Cameron Jason; Howard Kenneth A. An albumin-mediated cholesterol design-based strategy for tuning siRNA pharmacokinetics and gene silencing. Journal of Controlled Release (2016), 232, 143-151. [Abstract]
Abstract: Major challenges for the clinical translation of small interfering RNA (siRNA) include overcoming the poor plasma half-life, site-specific delivery and modulation of gene silencing. In this work, we exploit the intrinsic transport properties of human serum albumin to tune the blood circulatory half-life, hepatic accumulation and gene silencing; based on the number of siRNA cholesteryl modifications. We demonstrate by a gel shift assay a strong and specific affinity of recombinant human serum albumin (rHSA) towards cholesteryl-modified siRNA (Kd > 1 × 10− 7 M) dependent on number of modifications. The rHSA/siRNA complex exhibited reduced nuclease degradation and reduced induction of TNF-α production by human peripheral blood mononuclear cells. The increased solubility of heavily cholesteryl modified siRNA in the presence of rHSA facilitated duplex annealing and consequent interaction that allowed in vivo studies using multiple cholesteryl modifications. A structural-activity-based screen of in vitro EGFP-silencing was used to select optimal siRNA designs containing cholesteryl modifications within the sense strand that were used for in vivo studies. We demonstrate plasma half-life extension in NMRI mice from t1/2 12 min (naked) to t1/2 45 min (single cholesteryl) and t1/2 71 min (double cholesteryl) using fluorescent live bioimaging. The biodistribution showed increased accumulation in the liver for the double cholesteryl modified siRNA that correlated with an increase in hepatic Factor VII gene silencing of 28% (rHSA/siRNA) compared to 4% (naked siRNA) 6 days post-injection. This work presents a novel albumin-mediated cholesteryl design-based strategy for tuning pharmacokinetics and systemic gene silencing.
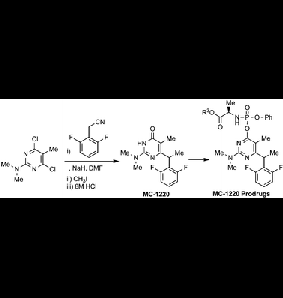
8. Loksha, Yasser M.; Pedersen, Erik B.; Loddo, Roberta; La Colla, Paolo. Synthesis and anti-HIV-1 evaluation of some novel MC-1220 analogs as non-nucleoside reverse transcriptase inhibitors. Archiv der Pharmazie (Weinheim, Germany) (2016), 349(5), 363-372. [Abstract]
Abstract: Some novel MC-1220 analogs were synthesized by a condensation of 4,6-dichloro-N-methylpyrimidin-2-amine derivs. and/or 4-chloro-6-methoxy-N,N,5-trimethylpyrimidin-2-amine with the sodium salt of 2,6-difluorophenylacetonitrile followed by treatment with aq. sodium hydroxide in methanol, alkylation, redn., halogenation, and/or acidic hydrolysis. All synthesized compds. were evaluated for their activity against HIV-1. The most active compd. in this study showed activity against HIV-1 comparable to that of MC-1220. The only difference in structure between compd. 7 and MC-1220 is a fluoro atom instead of a CH3 group.
7. Fontenete, Silvia; Guimaraes, Nuno; Wengel, Jesper; Azevedo Nuno, Filipe. Prediction of melting temperatures in fluorescence in situ hybridization (FISH) procedures using thermodynamic models. Critical reviews in biotechnology (2016), 36(3), 566-577. [Abstract]
Abstract: The thermodynamics and kinetics of DNA hybridization, i.e. the process of self-assembly of one, two or more complementary nucleic acid strands, has been studied for many years. The appearance of the nearest-neighbor model led to several theoretical and experimental papers on DNA thermodynamics that provide reasonably accurate thermodynamic information on nucleic acid duplexes and allow estimation of the melting temperature. Because there are no thermodynamic models specifically developed to predict the hybridization temperature of a probe used in a fluorescence in situ hybridization (FISH) procedure, the melting temperature is used as a reference, together with corrections for certain compounds that are used during FISH. However, the quantitative relation between melting and experimental FISH temperatures is poorly described. In this review, various models used to predict the melting temperature for rRNA targets, for DNA oligonucleotides and for nucleic acid mimics (chemically modified oligonucleotides), will be addressed in detail, together with a critical assessment of how this information should be used in FISH.
6. Geny Sylvain; Gissberg Olof; Pabon Y Vladimir; Zaghloul Eman M; Lundin Karin E; Smith C I Edvard; Moreno Pedro M D; Krzywkowski Tomasz; Nilsson Mats; Andersen Nicolai K; Isse Abdirisaq J; El-Madani Amro M; Lou Chenguang; Jørgensen Per T; Pedersen Erik B; Wengel Jesper; Anderson Brooke A; Hrdlicka Patrick J; Zain Rula. Next-generation bis-locked nucleic acids with stacking linker and 2′-glycylamino-LNA show enhanced DNA invasion into supercoiled duplexes. Nucleic Acids Research (2016), 44(5), 2007-2019. [Open Access]
Abstract: Targeting and invading double-stranded DNA with synthetic oligonucleotides under physiological conditions remain a challenge. Bis-locked nucleic acids (bisLNAs) are clamp-forming oligonucleotides able to invade into supercoiled DNA via combined Hoogsteen and Watson–Crick binding. To improve the bisLNA design, we investigated its mechanism of binding. Our results suggest that bisLNAs bind via Hoogsteen-arm first, followed by Watson–Crick arm invasion, initiated at the tail. Based on this proposed hybridization mechanism, we designed next-generation bisLNAs with a novel linker able to stack to adjacent nucleobases, a new strategy previously not applied for any type of clamp-constructs. Although the Hoogsteen-arm limits the invasion, upon incorporation of the stacking linker, bisLNA invasion is significantly more efficient than for non-clamp, or nucleotide-linker containing LNA-constructs. Further improvements were obtained by substituting LNA with 2′-glycylamino-LNA, contributing a positive charge. For regular bisLNAs a 14-nt tail significantly enhances invasion. However, when two stacking linkers were incorporated, tail-less bisLNAs were able to efficiently invade. Finally, successful targeting of plasmids inside bacteria clearly demonstrates that strand invasion can take place in a biologically relevant context.
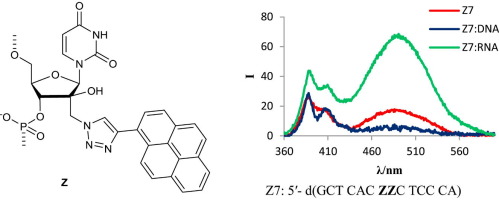
5. Gouda, Alaa S.; Amine, Mahasen S.; Pedersen, Erik B. Synthesis of new DNA G-quadruplex constructs with anthraquinone insertions and their anticoagulant activity. Helvetica Chimica Acta (2006), 99(2), 116-124. [Abstract]
Abstract: 1,4-Dihydroxyanthraquinone and 1,8-dihydroxyanthraquinone were alkylated with 3-bromopropan-1-ol and subsequently transformed into the corresponding DMT protected phosphoramidite building blocks for insertion into loops of the G-quadruplex of the thrombin binding aptamer (TBA). The 1,4-disubstituted anthraquinone linker led to a significant stabilization of the G-quadruplex structure upon replacing a T in each of two neighboring lateral TT loops and a 26.2° increase in thermal melting temperature was found. CD Spectra of the modified quadruplexes confirmed anti-parallel conformations in all cases under potassium buffer conditions as previously observed for TBA. Although the majority of the anthraquinone modified TBA analogues showed a decrease in clotting times in a fibrinogen clotting assay when compared to TBA, modified aptamers containing a 1,8-disubstituted anthraquinone linker replacing G8 or T9 in the TGT loop showed improved anticoagulant activities. Molecular modeling studies explained the increased thermal melting temperatures of anthraquinone-modified G-quadruplexes.
4. Fontenete, Silvia; Leite, Marina; Cappoen, Davie; Santos, Rita; Van Ginneken, Chris Figueiredo, Céu; Wengel, Jesper; Cos, Paul; Azevedo, Nuno F. Fluorescence in vivo hybridization (FIVH) for detection of Helicobacter pylori infection in a C57BL/6 mouse model. PLoS ONE (2016) 11(2): e0148353 [Open Access]
Abstract: In this study, we applied fluorescence in vivo hybridization (FIVH) using locked nucleic acid (LNA) probes targeting the bacterial rRNA gene for in vivo detection of H. pylori infecting the C57BL/6 mouse model. A previously designed Cy3_HP_LNA/2OMe_PS probe, complementary to a sequence of the H. pylori 16S rRNA gene, was used. First, the potential cytotoxicity and genotoxicity of the probe was assessed by commercial assays. Further, the performance of the probe for detecting H. pylori at different pH conditions was tested in vitro, using fluorescence in situ hybridization (FISH). Finally, the efficiency of FIVH to detect H. pylori SS1 strain in C57BL/6 infected mice was evaluated ex vivo in mucus samples, in cryosections and paraffin-embedded sections by epifluorescence and confocal microscopy.
3. Kumar, Pawan; Sharma, Pawan K.; Hansen, Jonas; Jedinak, Lukas; Reslow-Jacobsen, Charlotte; Hornum, Mick; Nielsen, Poul. Three new double-headed nucleotides with additional nucleobases connected to C-5 of pyrimidines; synthesis, duplex and triplex studies. Bioorganic & Medicinal Chemistry (2016), 24(4), 742-749. [Abstract]
Abstract: In the search for double-coding DNA-systems, three new pyrimidine nucleosides, each coded with an additional nucleobase anchored to the major groove face, are synthesized. Two of these building blocks carry a thymine at the 5-position of 2′-deoxyuridine through a methylene linker and a triazolomethylene linker, respectively. The third building block carries an adenine at the 6-position of pyrrolo-2′-deoxycytidine through a methylene linker. These double-headed nucleosides are introduced into oligonucleotides and their effects on the thermal stabilities of duplexes are studied. All studied double-headed nucleotide monomers reduce the thermal stability of the modified duplexes, which is partially compensated by using consecutive incorporations of the modified monomers or by flanking the new double-headed analogs with members of our former series containing propyne linkers. Also their potential in triplex-forming oligonucleotides is studied for two of the new double-headed nucleotides as well as the series of analogs with propyne linkers. The most stable triplexes are obtained with single incorporations of additional pyrimidine nucleobases connected via the propyne linker.
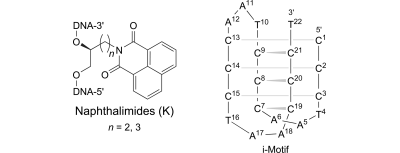
2. El-Sayed, Ahmed Ali; Pedersen, Erik B.; Khaireldin, Nahid Y. Thermal stability of modified i-motif oligonucleotides with naphthalimide intercalating nucleic acids. Helvetica Chimica Acta (2016), 99(1), 14-19. [Abstract]
Abstract: In continuation of our investigation of characteristics and thermodynamic properties of the i-motif 5′-d[(CCCTAA)3CCCT)] upon insertion of intercalating nucleotides into the cytosine-rich oligonucleotide, this article evaluates the stabilities of i-motif oligonucleotides upon insertion of naphthalimide (1H-benzo[de]isoquinoline-1,3(2H)-dione) as the intercalating nucleic acid. The stabilities of i-motif structures with inserted naphthalimide intercalating nucleotides were studied using UV melting temperatures (Tm) and circular dichroism spectra at different pH values and conditions (crowding and non-crowding). This study indicated a positive effect of the naphthalimide intercalating nucleotides on the stabilities of the i-motif structures compared to the wild-type structure which is in contrast to a previous observation for a pyrene-intercalating nucleotide showing a decrease in Tm values.
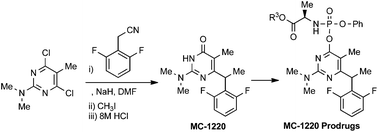
1. Loksha, Yasser M.; Pedersen, Erik B.; La Colla, Paolo; Loddo, Roberta. Facile synthesis of the NNRTI microbicide MC-1220 and synthesis of its phosphoramidate prodrugs. Organic & Biomolecular Chemistry (2016), 14(3), 940-946. [Abstract]
Abstract: A facile and novel synthetic route to MC-1220 was achieved by condensation of 4,6-dichloro-N,N-5-trimethylpyrimidin-2-amine (1) with the sodium salt of 2,6-difluorophenylacetonitrile, followed by methylation and strong acidic hydrolysis. The prodrugs of MC-1220 were synthesized by reaction of chlorophosphoramidate derivatives (7a–e) or α-acetobromoglucose with the sodium salt of MC-1220. The stability and anti-HIV-1 activity of phosphoramidate prodrugs turned out to be comparable to those of the parent drug MC-1220.