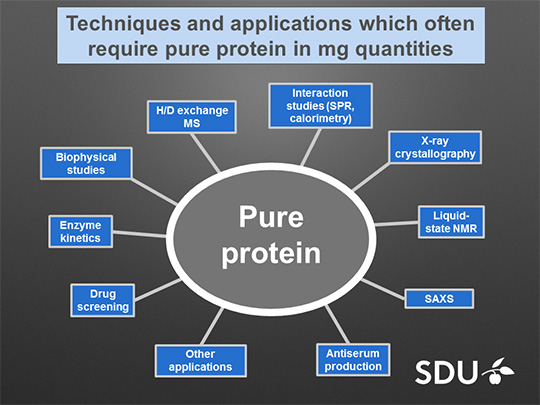
In many projects, obtaining more than 1 mg of pure protein from the authentic source (e.g. tissue or microorganism) or from recombinant (heterologous) expression in small-scale cell cultures can be a challenge. Insufficient amount of pure protein is often a limitation for applying several biotechnological, biochemical, pharmaceutical and medical research techniques.
Furthermore, for some protein structure determination methods, the protein must be labelled with specific isotopes during expression, and protein crystallography is normally incompatible with heterogeneity due to e.g. post-translational modifications. Other common challenges when working with proteins include low expression levels of soluble protein and misfolding of proteins and thus a demand for more efficient recombinant expression systems, up-scaling, or refolding in vitro.
After producing the protein in a recombinant expression system, it must be purified from the host cells or from the cell culture medium (if secreted bythe cells). Obtaining a highly pure target protein may require several purification steps where it is separated from other proteins based on solubility, biological activity, charge, or size and shape.
Thus, since the purification procedure is protein-dependent, it can be a time consuming and demanding task to make a preparation of highly pure protein. In general, purification of proteins often requires a long process of optimization to obtain a reliable and suitable protocol.